The Complete Guide to Understanding Aging: 9 Hallmarks and Breakthrough Interventions
Aging affects every living organism on Earth, yet scientists have only recently begun to decode its fundamental mechanisms. This comprehensive guide explores the groundbreaking framework of the nine hallmarks of aging, revolutionary interventions that may slow the aging process, and practical strategies you can implement today to support healthy aging and longevity.

Why Understanding Aging Matters More Than Ever
The science of aging has transformed dramatically over the past decade. Rather than viewing aging as an inevitable decline, researchers now understand it as a complex biological process with identifiable markers that can potentially be influenced. This shift in perspective opens extraordinary possibilities for extending not just lifespan, but healthspan—the years we live in good health.
Leading institutions worldwide have contributed to this evolving understanding. The SENS Research Foundation, under the guidance of pioneering scientist Aubrey de Grey, has championed the approach of treating aging as a collection of damage that accumulates over time. Similarly, Maria Blasco, Director at the Spanish National Cancer Research Centre, has made groundbreaking contributions that reshape how we approach aging interventions.
The Landmark Discovery: Nine Hallmarks of Aging
In 2013, a revolutionary paper published in the prestigious journal Cell transformed our understanding of aging. Maria Blasco and her collaborators identified nine interconnected hallmarks that characterize the aging process across different species, particularly in mammals. This landmark study has been cited over 4,000 times and serves as the foundation for modern aging research.
What Qualifies as a Hallmark of Aging?
For a biological process to be considered a hallmark of aging, it must meet three critical criteria:
- Natural Occurrence: The hallmark must appear during normal aging processes
- Acceleration Test: When the hallmark worsens, aging accelerates
- Intervention Response: Improving the hallmark leads to delayed aging and enhanced healthspan
The Nine Hallmarks of Aging Explained
Understanding these nine hallmarks provides a roadmap for targeting aging at its roots. Each hallmark represents a distinct aspect of cellular and molecular dysfunction:
Primary Hallmarks: The Root Causes
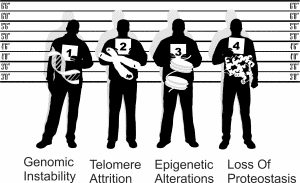
Four hallmarks serve as the primary drivers of cellular damage in aging:
1. Genomic Instability
Your DNA faces constant assault from environmental factors, radiation, and cellular processes. Over time, mutations accumulate, compromising cellular function. Think of it as typos accumulating in an instruction manual—eventually, the instructions become unreadable.
2. Telomere Attrition
Telomeres act as protective caps on chromosomes, similar to the plastic tips on shoelaces. With each cell division, telomeres shorten. When they become critically short, cells can no longer divide properly, contributing to aging symptoms.
3. Epigenetic Alterations
Your genes remain the same throughout life, but how they express changes dramatically with aging. Epigenetic modifications act like switches, turning genes on or off. Age-related changes in these patterns disrupt normal cellular function.
4. Loss of Proteostasis
Cells must constantly produce, fold, and dispose of proteins correctly. During aging, this quality control system fails, leading to accumulation of damaged proteins that impair cellular function.
Antagonistic Hallmarks: The Body's Compensatory Responses
Three hallmarks represent the body's attempts to compensate for primary damage, which initially help but eventually contribute to aging:
5. Deregulated Nutrient Sensing
Cells lose their ability to properly respond to nutrients. Key pathways like insulin signaling and mTOR become dysfunctional, affecting metabolism and cellular growth.
6. Mitochondrial Dysfunction
Mitochondria, the cellular powerhouses, decline in efficiency. This leads to reduced energy production and increased oxidative stress, accelerating aging throughout the body.
7. Cellular Senescence
Damaged cells enter a zombie-like state—they stop dividing but refuse to die. These senescent cells secrete inflammatory factors that damage surrounding healthy tissue.
Integrative Hallmarks: The Visible Signs of Aging
Two hallmarks represent the culmination of all other damage, manifesting as the visible signs of aging:
8. Stem Cell Exhaustion
Your body's repair system depends on stem cells. As these regenerative cells decline in number and function, tissues lose their ability to maintain and repair themselves.
9. Altered Intercellular Communication
Cells must communicate effectively to coordinate bodily functions. Aging disrupts these communication networks, leading to chronic inflammation and reduced tissue function.
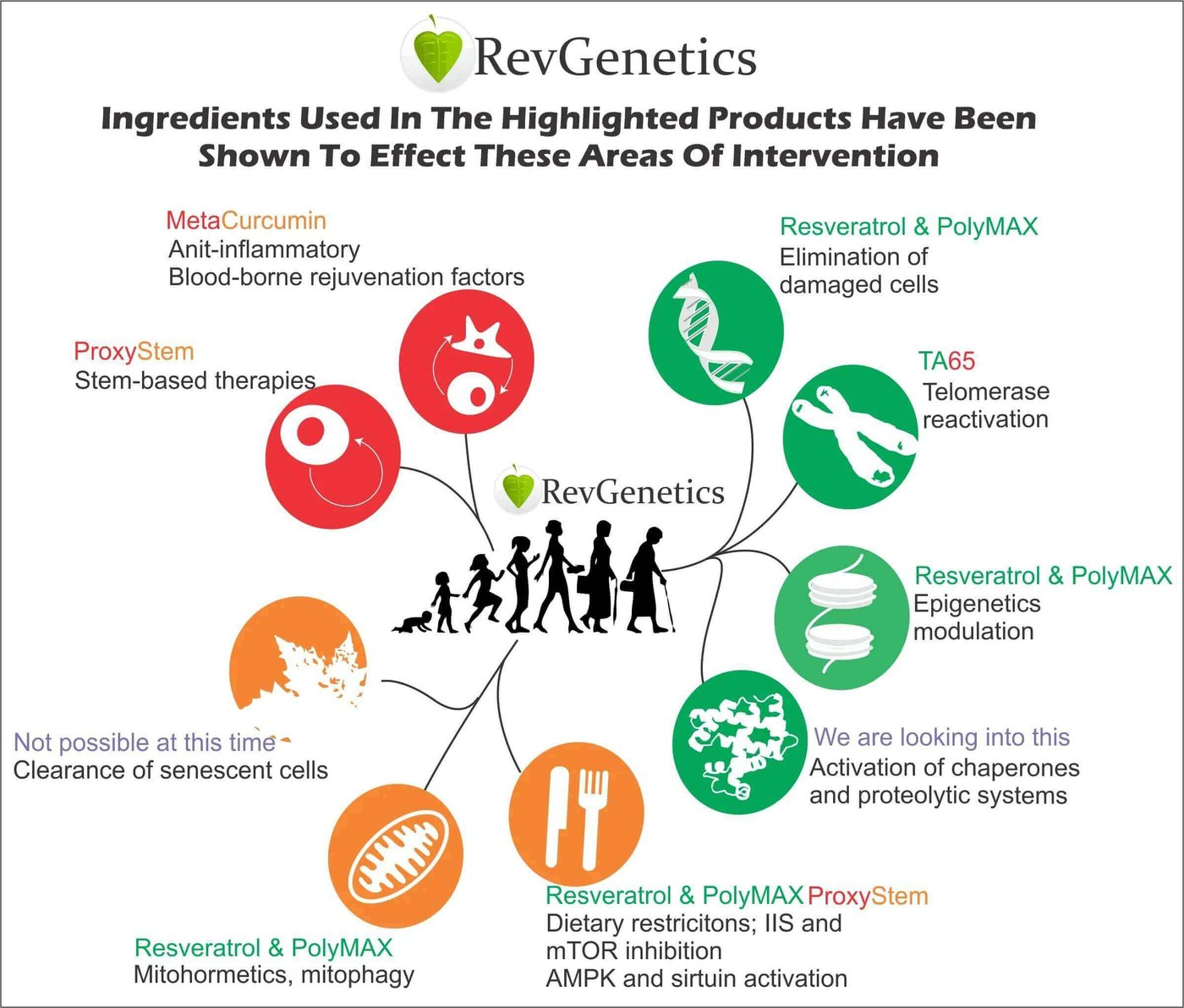
Revolutionary Interventions for Healthy Aging

The identification of aging hallmarks has opened new avenues for intervention. Scientists have developed targeted approaches to address each hallmark, offering hope for extending healthspan:
Cutting-Edge Intervention Strategies
1. Stem Cell Rejuvenation
Supporting stem cell health maintains the body's regenerative capacity. While medical stem cell therapies continue advancing, nutritional support for existing stem cells shows promise. Compounds that protect stem cell populations help preserve regenerative potential throughout aging.
2. Inflammation Control
Chronic inflammation accelerates virtually every aspect of aging. Natural anti-inflammatory compounds like curcumin have shown remarkable ability to modulate inflammatory pathways. Advanced formulations enhance bioavailability, maximizing these protective effects.
3. Senescent Cell Clearance
Removing zombie-like senescent cells rejuvenates tissues. Senolytic compounds selectively target these problematic cells while sparing healthy ones. Fisetin, a natural flavonoid, demonstrates powerful senolytic properties exceeding those of resveratrol or quercetin.
4. Telomerase Activation
Lengthening telomeres may reset the cellular aging clock. While medical approaches remain experimental, certain natural compounds show ability to activate telomerase, the enzyme that maintains telomere length. This represents one of the most exciting frontiers in aging research.
5. Epigenetic Modulation
Resetting epigenetic patterns to a more youthful state shows tremendous promise. Sirtuins, a family of proteins activated by compounds like resveratrol, play crucial roles in maintaining healthy epigenetic patterns throughout aging.
6. Protein Quality Control
Supporting cellular protein management systems helps prevent accumulation of damaged proteins. Activation of chaperone proteins and cellular cleanup mechanisms maintains proteostasis during aging.
7. Metabolic Optimization
Dietary restriction mimetics activate longevity pathways without requiring severe calorie restriction. Key targets include mTOR inhibition and AMPK activation, both achievable through specific nutritional interventions. These pathways profoundly influence the aging process at the cellular level.
8. Mitochondrial Support
Enhancing mitochondrial function through hormesis—mild stress that strengthens cellular defenses—improves energy production. Supporting mitophagy, the selective removal of damaged mitochondria, maintains cellular energy throughout aging.
9. Cellular Communication Enhancement
Restoring youthful cellular communication patterns reduces age-related dysfunction. This involves both reducing inflammatory signals and enhancing beneficial growth factors.
Practical Implementation: Your Aging Intervention Strategy
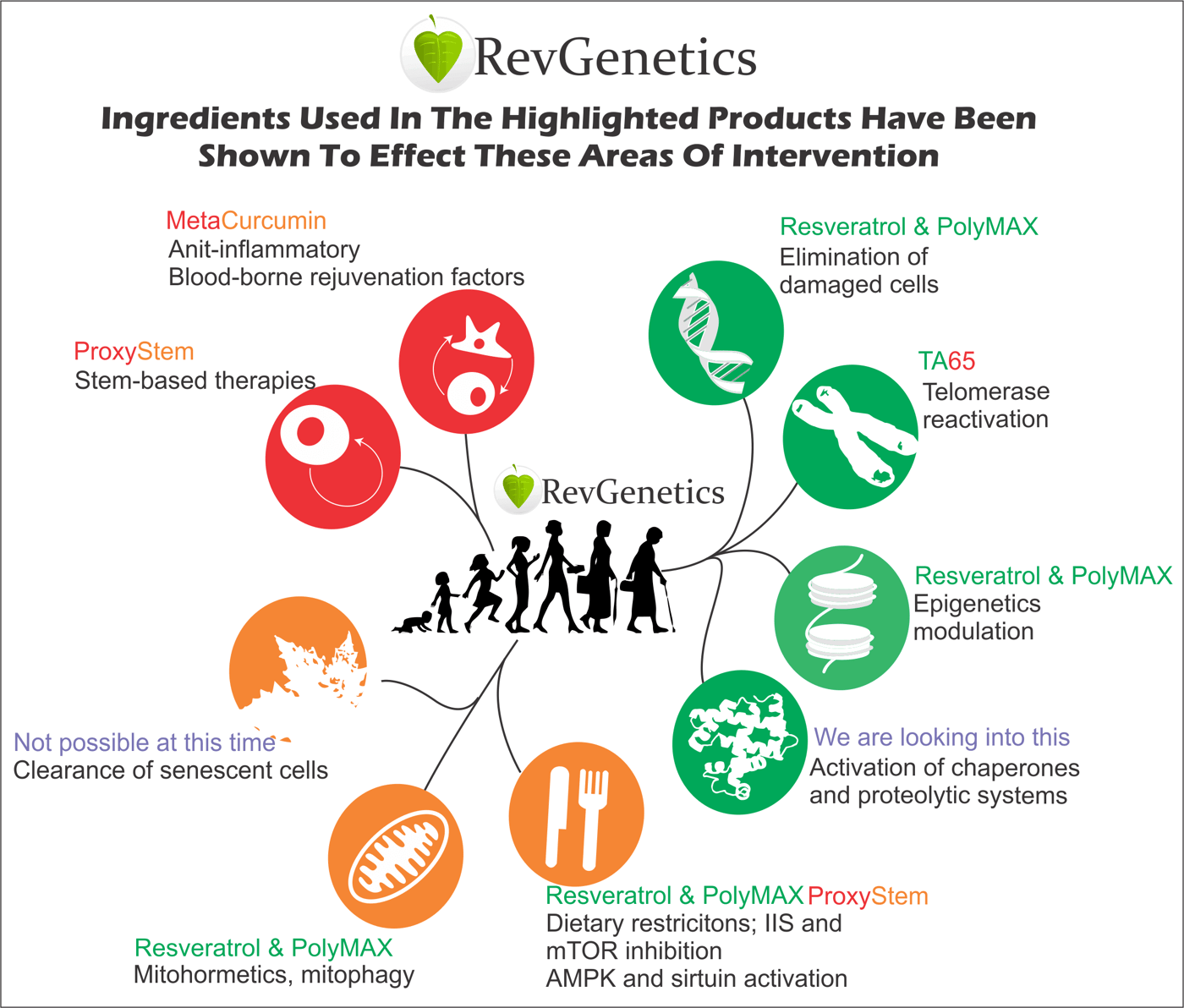
Translating scientific discoveries into practical strategies empowers individuals to take control of their aging process. Here's how specific interventions map to available solutions:
Comprehensive Intervention Approach
For Stem Cell Support: Specialized formulations like stem cell support complexes help maintain the regenerative capacity crucial for healthy aging. These work by protecting existing stem cell populations and supporting their optimal function.
For Inflammation Management: Advanced curcumin formulations address the chronic inflammation that accelerates aging. Liquid capsule technology enhances absorption, maximizing anti-inflammatory benefits throughout the body.
For Damaged Cell Elimination: Resveratrol formulations support activation of tumor suppressor genes p16 and p53, helping identify and remove damaged cells before they become problematic.
For Telomere Maintenance: Telomerase activators represent a breakthrough in addressing cellular aging at its foundation. These compounds have demonstrated safety in human studies and show promise for maintaining telomere length.
For Epigenetic Balance: Sirtuin activators like resveratrol modulate epigenetic targets linked to longevity. This approach addresses aging at the gene expression level, potentially resetting cellular age.
For Metabolic Optimization: Combining resveratrol with curcumin creates synergistic effects on longevity pathways. Adding intermittent fasting amplifies these benefits, activating cellular renewal processes.
For Mitochondrial Health: Sirtuin activation through resveratrol improves mitochondrial respiration via the PGC-1α pathway. This enhances cellular energy production, combating age-related fatigue.
For Senescent Cell Removal: High-dose fisetin formulations (500mg) provide powerful senolytic activity. This natural compound outperforms other flavonoids in clearing senescent cells, rejuvenating tissues throughout the aging process.
The Future of Aging Science
We stand at an unprecedented moment in human history. For the first time, we understand aging not as an inevitable decline but as a biological process with identifiable targets for intervention. This knowledge transforms aging from a passive experience to an active area where informed choices can make profound differences.
Research continues advancing rapidly. New discoveries regularly refine our understanding of aging mechanisms and reveal novel intervention strategies. What seemed like science fiction merely decades ago now enters clinical trials and practical application.
The convergence of multiple scientific disciplines—genetics, molecular biology, bioinformatics, and nutritional science—accelerates progress exponentially. Each breakthrough builds upon previous discoveries, creating a compound effect that promises even more dramatic advances ahead.
Taking Action: Your Personal Aging Strategy
Knowledge without action remains merely potential. Understanding the hallmarks of aging empowers you to make informed decisions about your health trajectory. Consider these actionable steps:
- Assess Your Current State: Understanding where you are helps determine where to focus efforts
- Prioritize Interventions: Not all hallmarks require equal attention—identify your primary concerns
- Implement Gradually: Sustainable changes trump dramatic short-term efforts
- Monitor Progress: Track improvements in energy, cognitive function, and overall wellbeing
- Stay Informed: Aging science evolves rapidly—remain open to new discoveries
Remember that addressing aging comprehensively yields the greatest benefits. While targeting individual hallmarks helps, the interconnected nature of aging mechanisms means comprehensive approaches produce synergistic effects exceeding the sum of individual interventions.
Conclusion: Embracing the Possibilities
The science of aging has reached an inflection point. We now possess both the understanding and tools to influence our biological age meaningfully. The nine hallmarks framework provides a scientific roadmap, while emerging interventions offer practical solutions.
This represents more than extending lifespan—it's about enhancing the quality of every year lived. Imagine maintaining youthful energy, mental clarity, and physical capability decades longer than previous generations thought possible. This vision moves closer to reality with each scientific advance.
Your journey toward healthy aging begins with understanding these fundamental mechanisms. Armed with this knowledge, you can make informed choices that support cellular health, maintain regenerative capacity, and optimize biological function throughout life. The future of aging is bright, and it starts with the decisions you make today.
Frequently Asked Questions About Aging
What exactly causes aging at the cellular level?
Aging results from accumulated cellular damage across nine interconnected hallmarks. The four primary causes include genomic instability (DNA damage), telomere shortening (chromosome cap erosion), epigenetic changes (gene expression alterations), and protein dysfunction. These trigger compensatory responses that eventually fail, leading to visible aging. Understanding these mechanisms opens possibilities for targeted interventions that may slow or partially reverse age-related decline.
Can aging actually be reversed or just slowed down?
Current science suggests both slowing and partial reversal are possible. While complete age reversal remains theoretical, specific hallmarks like epigenetic patterns and telomere length show reversibility in laboratory settings. Senolytic compounds can remove aged cells, effectively rejuvenating tissues. Practical interventions today focus on slowing aging and reversing specific damage markers. Future breakthroughs may enable more comprehensive rejuvenation.
Which aging interventions show the most promise currently?
Several interventions demonstrate exceptional promise: Senolytics (like fisetin) for removing damaged cells, telomerase activators for maintaining chromosome integrity, sirtuin activators (resveratrol) for metabolic optimization, and anti-inflammatory compounds (curcumin) for reducing chronic inflammation. Combining multiple approaches creates synergistic effects. Dietary restriction mimetics and mitochondrial support also show significant potential for extending healthspan.
How do I know which aging hallmarks affect me most?
While comprehensive testing remains limited, certain signs indicate specific hallmark involvement. Low energy suggests mitochondrial dysfunction, frequent infections indicate stem cell exhaustion, and chronic inflammation points to senescent cell accumulation. Age itself provides clues—telomere attrition accelerates after 60, while protein dysfunction often appears earlier. Consulting with longevity-focused healthcare providers can help identify your primary aging drivers through biomarker analysis.
What's the difference between chronological and biological aging?
Chronological age simply counts years since birth, while biological age reflects your body's actual functional state. Two 50-year-olds might have vastly different biological ages based on lifestyle, genetics, and environmental factors. Biological aging can be influenced through targeted interventions addressing the nine hallmarks. Advanced testing can now estimate biological age through DNA methylation patterns, telomere length, and other biomarkers, offering insights into intervention effectiveness.
Should I target all nine hallmarks of aging simultaneously?
While comprehensive approaches yield best results, targeting all hallmarks simultaneously isn't always practical or necessary. Focus on primary hallmarks (genomic instability, telomere attrition, epigenetic alterations, proteostasis) provides upstream benefits affecting other hallmarks. Start with 2-3 key interventions based on your priorities, then expand gradually. Many interventions address multiple hallmarks—resveratrol, for instance, impacts epigenetics, mitochondrial function, and inflammation simultaneously.
How long before I see results from aging interventions?
Timeline varies by intervention and individual factors. Energy improvements from mitochondrial support often appear within weeks. Inflammation reduction typically shows benefits in 1-3 months. Telomere lengthening and epigenetic changes require 6-12 months of consistent intervention. Senolytic effects may be noticeable within months as damaged cells clear. Remember, aging interventions work cumulatively—consistency matters more than intensity for long-term benefits.
Are aging interventions safe for long-term use?
Most evidence-based aging interventions demonstrate excellent long-term safety profiles. Natural compounds like resveratrol, curcumin, and fisetin have been consumed in foods for millennia. Clinical studies confirm safety at therapeutic doses. However, individual responses vary—start with lower doses and increase gradually. Some interventions work best cyclically rather than continuously. Always consult healthcare providers, especially if taking medications or managing health conditions.
What role does lifestyle play compared to supplements in aging?
Lifestyle forms the foundation of healthy aging—supplements enhance but cannot replace good habits. Exercise, quality sleep, stress management, and whole food nutrition address multiple aging hallmarks naturally. Supplements provide targeted support for specific hallmarks that lifestyle alone may not adequately address. The most effective approach combines both: optimize lifestyle factors while using evidence-based supplements to target specific aging mechanisms. This synergy produces results exceeding either approach alone.
How does aging research translate to practical daily choices?
Understanding aging science empowers informed daily decisions. Choose foods rich in senolytic compounds (berries, green tea), practice intermittent fasting to activate longevity pathways, prioritize sleep for cellular repair, and manage stress to reduce inflammation. Select supplements based on scientific evidence rather than marketing claims. Time interventions strategically—take mitochondrial support morning for energy, senolytics cyclically for cellular cleanup. Small, science-based choices compound into significant healthspan extensions over time.